A SEARCH FOR MAXIMUM SPECIES ABUNDANCES IN ECOLOGICAL COMMUNITIES UNDER CONDITIONAL DIVERSITY OPTIMIZATION
V.L. Alexeyev and A.P. Levich*
Department of Zoology of Vertebrates
and General Ecology
Biology Faculty
Moscow State University
Vorobyovy Gory
Moscow 119899
Russia
(E-mail:
 )
)
We study a multi-species community of autotrophic microorganisms which grow in a batch culture regime with several perfectly complementary resources. A basic hypothesis is that a stationary phase of the polyculture corresponds to a maximum diversity under the constraints having the meaning of matter conservation laws. The corresponding conditional extremum problem is studied in detail. It is shown that a unique solution to this problem — a “species structure formula” — adequately describes the experimental data. We prove a number of strict statements concerning the domain of definition and maxima of the obtained solutions. These statements find an adequate interpretation as limitation principles in ecology and in the problems of community structure control.
1. Introduction. The idea that the problem of finding a stationary state of a complex physical system can be reduced to that of conditional optimization of a certain goal function has apparently occurred to Boltzmann and, independently, to Gibbs when they studied the problems of statistical mechanics. Gibbs (1902) has shown that the canonical distribution function is obtained when the entropy is maximized under the condition of a specified average energy. Afterwards this idea acquired applications in other areas of physics (Levine and Tribus, 1979), as well as in information theory (Brillouin, 1963) and the theory of self-organized systems (Haken, 1988).
A general feature of problems where this approach is applicable is that a detailed description of a system under consideration, for instance, in the form of differential equations, is either extremely complicated, or simply impossible due to lack of understanding of internal processes occurring in the system.
Quite naturally, the researchers dealing with biological systems, which are as a rule much more complex than physical ones, could not help paying attention to the above method. It is sufficient to mention some recent works (containing numerous references to earlier papers) dedicated to application of the conditional optimization method to the problems of biochemistry (Schuster and Heinrich, 1991; Wilhelm et al., 1994), breath (Karpouzas, 1986) and embryogenesis (Lewis et al., 1988).
We apply conditional optimization for finding species abundances distribution at a stationary phase of batch growth of a multispecies community of autotrophic microorganisms which consume several perfectly complementary resources.
There is a large amount of works which consider the development of a multispecies community, growing on a multicomponent substrate from both theoretical and experimental viewpoints (e.g., Armstrong and McGehee, 1980; Butler and Wolkowicz, 1987; Fredrickson and Stephanopoulos, 1981; Leon and Tumpson, 1975; Tilman, 1982; Waltman et al., 1980; Ballyk and Wolkowicz, 1995; Degermendzhy et al., 1989; Vavilin et al., 1993). We consider the situation of batch growth, i.e., the case of unrenewable resources. A community subject to batch culture is placed in a medium with a certain amount of nutrients and then grows (without addition or removal of nutrients and microorganisms in any artificial way) up to a stationary phase, such that cell fission of all species entering into the community is stopped (the lysis is usually insignificant and may be neglected at the growth stage). If the experiment is continued, then, after some time spent in a stationary state, the community naturally begins to degrade.
We study just the stationary phase of growth. There exists a sufficient amount of natural ecosystems whose functioning is close to the batch culture process. One of such examples is provided by ponds and lakes where the deposits of biogenic elements are added mainly in spring, when enriched waters from below the thermocline are mixed with the water bulk of the photic layer (Odum, 1983). Therefore, if for some period the phytoplankton eating-out by animal plankton and fish can be neglected, one can assume with good accuracy that the phytoplankton community spends a considerable part of this period of time at a stationary phase. Moreover, it is just the stationary phase of a culture that is of basic interest in various biotechnological applications, since this is the stage of ``harvesting''.
Modelling the growth dynamics of an batch culture by a set of differential equations encounters a number of difficulties. Above all, the set of variables of this set of differential equations should contain not only the abundances (or biomass concentrations) of the species and the absolute amounts (or concentrations) of biogenic elements in the environment, but also the concentrations of nutrients inside the cells of each species (the cell quotas according to Droop's (1968, 1973) terminology). Besides, numerous experiments with batch culture of phytoplankton (Droop, 1983; Levich et al., 1986a) have shown that the process of growth of cell population abundances due to biogenic substrate consumption can be naturally divided into three stages: A — biogen accumulation in the cells, without their growth in number; B — cell fission due to environmental substance consumption; C — cell fission without consuming the environmental substance, at the expense of inner nutrient deposits. And, strictly speaking, each stage requires a set of differential equations of its own to be written, so that afterwards the solution sewing problem ought to be solved. And even if we, neglecting some effects, write down a certain set of differential equations that describes the whole process, even the simplest system of this sort for the case of a culture of w species consuming m resources, contains m +w +mw equations and about 2w +4mw parameters (Jørgensen, 1980; Levich and Lichman, 1992), so that even for very simple situations the problem of parameter identification with the experimental data becomes extremely difficult.
Keeping in mind these problems, we seek a stationary phase of a polyculture with the aid of the conditional optimization method. We believe that at a stationary stage a community possesses maximum diversity under natural constraints due to matter conservation laws. In Section 2 we discuss the subject in more detail. In Section 3 we carry out an analytic study of an extremal problem. It turns out that the extremal of the problem — the “Species Structure Formula” — adequately describes the experimental data and also lets one utter some general statements concerning the problems of algocoenosis structure control and the limitation principles on ecology. These topics are discussed in Section 4. In the last section we present some general considerations on the applications of the conditional optimization method in biology.
2. General Considerations. We consider the situation when a multispecies community of organisms is placed in a medium containing different nutrients necessary for the growth of the community species. We assume that these nutrients cannot replace one another since they realize different functions with respect to the growth. That is, these nutrients are perfectly complementary according to Leon and Tumpson's (1975) classification. As the community grows, neither the resources are renewed, nor the organisms are extracted. Such a situation may be exemplified by batch culture of microorganisms under such conditions that the natural lysis in the process of growth may be neglected. After some time, the abundance growth of all species entering into the community ceases. This is caused by the unrenewable nature of the resources and is connected with the fact that the inner content of one or several nutrients in a cell (the cell quota) reaches a certain critical value, such that its further lowering makes cell fission impossible (Ketchum, 1939; Kuenzler and Ketchum, 1962; Droop, 1983). This critical quantity, called the minimal cell quota, has a value of its own for each species and also depends on the nutrient. The community resides in a stationary phase for some time and then, if no resources are added, there occurs a natural degradation stage; later on the species die out.
We assume that, at the stationary growth stage, the species numbers distribution in a community of microorganisms consisting of w species and consuming m resources, is a solution to the following conditional maximum problem:
 (1)
(1)
here xi is the abundance of the i-th species,  . The constraints in the form of inequalities are just the matter conservation laws, where
. The constraints in the form of inequalities are just the matter conservation laws, where  is the content of the k-th substance in a cell of the i-th species at the growth halting instant,
is the content of the k-th substance in a cell of the i-th species at the growth halting instant,  is the same quantity at the beginning of the experiment,
is the same quantity at the beginning of the experiment,  is the content of the k-th substance in the environment at the beginning of the experiment and xi0 is the initial abundance of the i-th species. Lk is a notation introduced in order to simplify the form of the right-hand side of the inequalities. The constraints have been written in the form of inequalities because the growth stopping does not at all mean that all m nutrients have been entirely consumed, i.e., have got inside the cells. For growth stopping it is sufficient to exhaust at least one of the nutrients (for more details see Section 4).
is the content of the k-th substance in the environment at the beginning of the experiment and xi0 is the initial abundance of the i-th species. Lk is a notation introduced in order to simplify the form of the right-hand side of the inequalities. The constraints have been written in the form of inequalities because the growth stopping does not at all mean that all m nutrients have been entirely consumed, i.e., have got inside the cells. For growth stopping it is sufficient to exhaust at least one of the nutrients (for more details see Section 4).
The function  is the well-known diversity index, used to characterize the diversity per individual in a community of organisms (MacArthur, 1955; Margalef, 1951; Ludwig and Reynolds, 1988). Thus the function H(
is the well-known diversity index, used to characterize the diversity per individual in a community of organisms (MacArthur, 1955; Margalef, 1951; Ludwig and Reynolds, 1988). Thus the function H( ). may be treated as a diversity indicator for the community as a whole. This choice of the function to be extremized is connected with the fact that an approach similar to ours, with a like goal function, has shown a good agreement with experimental data in other situations (Prits, 1974; Lurie et al., 1983). Besides, a community in a stationary phase should possess a maximum stability, while, in the opinion of some researchers, this is closely related to maximum diversity (Woodwell and Smith, 1969).
). may be treated as a diversity indicator for the community as a whole. This choice of the function to be extremized is connected with the fact that an approach similar to ours, with a like goal function, has shown a good agreement with experimental data in other situations (Prits, 1974; Lurie et al., 1983). Besides, a community in a stationary phase should possess a maximum stability, while, in the opinion of some researchers, this is closely related to maximum diversity (Woodwell and Smith, 1969).
3. Mathematical analysis and results. Here we will present the main results of studying the problem (1). We do our best to reduce the proofs and try to remove the technical part of the proofs to the Appendices. In addition, where possible, we always briefly explain the biological meaning of the results being obtained, postponing a detailed discussion till Section 4.
Here and henceforth, without mentioning that each time, we will assume that the following conditions, naturally arising from the sense of the problem, are fulfilled:

The condition  is imposed because situations when the number of species is greater than or equal to the number of resources, is most generic. The condition upon rank
is imposed because situations when the number of species is greater than or equal to the number of resources, is most generic. The condition upon rank is introduced because the species in (1) virtually differ from one another only on their demands
is introduced because the species in (1) virtually differ from one another only on their demands  , so that the requirement that the rank of the demands matrix be maximum means that all w species are indeed different.
, so that the requirement that the rank of the demands matrix be maximum means that all w species are indeed different.
Theorem 1 (Existence and Uniqueness Theorem). Given any vector  IR° m+ = =
IR° m+ = = , a solution to the problem (1) exists, is unique and has the form
, a solution to the problem (1) exists, is unique and has the form
 , (2)
, (2)
where  , while n and
, while n and  form a solution to the following set of equations
form a solution to the following set of equations
 (3)
(3)
Proof: here we would like to present just a sketch of a proof (see a full version in Levich et al., 1994.)
The existence of a solution follows from the continuity of H( ) (we assume that xln x =0 at x =0) and the compactness of the set specified by the constraint inequalities in (1).
) (we assume that xln x =0 at x =0) and the compactness of the set specified by the constraint inequalities in (1).
Lemma. for each  IR° m+ a maximum of H(
IR° m+ a maximum of H( ) in (1) is achieved at
) in (1) is achieved at 
A proof of the lemma is given in Appendix 1.
H( ) is a smooth function on the domain IR° w+
) is a smooth function on the domain IR° w+ IRm
IRm , and we may apply the standard Lagrange method of solving extremal problems constrained by inequalities (Luenberger, 1984). After that we arrive at (3).
, and we may apply the standard Lagrange method of solving extremal problems constrained by inequalities (Luenberger, 1984). After that we arrive at (3).
The uniqueness of the solution follows from the convexity of H( ).
).
Corollary 1: if  is solution (1), then
is solution (1), then  , i.e., the abundances of all species of the community at a stationary stage of the polyculture growth are positive, or, in other words, all the species which were present at the initial stage, are present as well at the stationary stage.
, i.e., the abundances of all species of the community at a stationary stage of the polyculture growth are positive, or, in other words, all the species which were present at the initial stage, are present as well at the stationary stage.
Corollary 2: the equality  holds for (2), where n is found from (3), so that n has the meaning of the total abundance.
holds for (2), where n is found from (3), so that n has the meaning of the total abundance.
Let us discuss the ecological meaning of the Lagrange multipliers.
If we have single resource (m = 1) the species of the community are assigned numbers according to decreasing abundances, then the species structure formula (2) is nothing else than the so-called rank distribution of abundances. For example, under a linear distribution of the quotas qi in the single limiting resource, an exponential dependence of the abundance of a species on its rank takes place; under a slowly varying distribution of quotas, approximated by a logarithmic dependence on the rank, the rank dependence of the species abundances is hyperbolic. The Lagrange multipliers are the rank disribution parameters and reflect the rate (abruptness, smoothness, homogeneity, uniformity, etc.) of the decrease of abundances from group to group, or, which is the same, the degree of dominance of groups with high abundances. These parameters are called species diversity indices and are conventionally calculated by approximate formulae (Levich, 1980):  for exponential rank distributions (Margalef, 1951),
for exponential rank distributions (Margalef, 1951),  for hyperbolic distributions with b= 2 (Menhinik, 1964) or
for hyperbolic distributions with b= 2 (Menhinik, 1964) or  for b= 1 (Odum et al., 1960).
for b= 1 (Odum et al., 1960).
Another interpretation is connected with the dimension analysis for the Lagrange multipliers. As the exponent of the exponential function in the species structure formula (3) is dimensionless, the dimension of the multiplier lk is inverse with respect to that of the demands  . That is, the quantity 1/lk may be proportional to the community-averaged demand for the resource k, having the same dimension. (We would like to note that, in the case of thermodynamics of perfect gas, it turns out that, under a fixed energy, 1/l = T, where T is proportional to the molecules' mean kinetic energy.)
. That is, the quantity 1/lk may be proportional to the community-averaged demand for the resource k, having the same dimension. (We would like to note that, in the case of thermodynamics of perfect gas, it turns out that, under a fixed energy, 1/l = T, where T is proportional to the molecules' mean kinetic energy.)
One more interpretation is connected with the variational modelling theorem which states that

(Intrilligator, 1971), i.e., the Lagrange multiplier is the entropy changing rate due to changes in the resource k, therefore it may be interpreted as the "strength of the effect" of the factor k upon the "goal function" H. Namely, if a small amount of the resource DLk is added to the environment, i.e., the limitation is weakened, then the diversity H* will be changed by lk DLk. In particular, if lk = 0, then, for a given resource vector  , the amount of the k-th resource causes no constraint upon the system diversity.
, the amount of the k-th resource causes no constraint upon the system diversity.
It turns out that, if lk = 0, then the k-th resource has no effect not only upon the integral characteristic, the diversity of the whole polyculture, but also upon xi,  , the polyculture species abundances at the stage of stationary growth. Prior to proving a rigorous result, let us discuss this in some more detail.
, the polyculture species abundances at the stage of stationary growth. Prior to proving a rigorous result, let us discuss this in some more detail.
If, for some specified  , we solve the system (3) and substitute the obtained solution (2) into constraint inequalities of the problem (1), then some of them will become equalities, while others will become strict inequalities. That means that some of the nutrients have been entirely consumed and others not. Herewith, as follows from the system (3), if some k-th (
, we solve the system (3) and substitute the obtained solution (2) into constraint inequalities of the problem (1), then some of them will become equalities, while others will become strict inequalities. That means that some of the nutrients have been entirely consumed and others not. Herewith, as follows from the system (3), if some k-th ( ) substance is not entirely consumed, i.e.,
) substance is not entirely consumed, i.e.,  , then lk = 0 and therefore we obtain from Eq. (2) that xi,
, then lk = 0 and therefore we obtain from Eq. (2) that xi,  are independent of
are independent of  ,
,  . In other words, if a nutrient is not entirely consumed from the environment, i.e., it has not been the cause of growth halting, then the abundances xi,
. In other words, if a nutrient is not entirely consumed from the environment, i.e., it has not been the cause of growth halting, then the abundances xi,  . of the polyculture species at the stationary growth stage do not depend on the cellular quota values for this nutrient. This reasoning leads us in Section 4 to a limitation conception which generalizes the classical ones. And now we will obtain the corresponding theorem (Theorem 3). A reader who is not interested in the technical details of its proof, may at once pass to its formulation without sacrifice of understanding.
. of the polyculture species at the stationary growth stage do not depend on the cellular quota values for this nutrient. This reasoning leads us in Section 4 to a limitation conception which generalizes the classical ones. And now we will obtain the corresponding theorem (Theorem 3). A reader who is not interested in the technical details of its proof, may at once pass to its formulation without sacrifice of understanding.
In order to formulate and prove further results, we will investigate the problem
 (4)
(4)
where  and the matrix
and the matrix  is obtained from the matrix
is obtained from the matrix 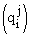 of the problem (1) by removing columns numbered
of the problem (1) by removing columns numbered 
We assume that  belongs to the interior of the cone
belongs to the interior of the cone  , where
, where  is the number of elements in J and
is the number of elements in J and  In a way similar to the lemma from Theorem 1, one can show that a maximum of H(
In a way similar to the lemma from Theorem 1, one can show that a maximum of H( ) in (4) is reached for such
) in (4) is reached for such  at
at  . Now, before applying Lagrange's method for solving extremal problems with equalities (Luenberger, 1984), we would like to note that, as follows from the first equation (3), for the problem (1) with m =1, the quantity
. Now, before applying Lagrange's method for solving extremal problems with equalities (Luenberger, 1984), we would like to note that, as follows from the first equation (3), for the problem (1) with m =1, the quantity  and does not depend on L, therefore, according to the second equation (3), the equality
and does not depend on L, therefore, according to the second equation (3), the equality 
 will always hold on the solution. Thus, for m =1, (1) and (4) are equivalent. Therefore we will assume in (4)
will always hold on the solution. Thus, for m =1, (1) and (4) are equivalent. Therefore we will assume in (4)  , and, since for all such J an investigation of (4) is carried out using the same scheme, we assume for simplicity that in (4)
, and, since for all such J an investigation of (4) is carried out using the same scheme, we assume for simplicity that in (4)  .
.
Then one can show (Levich et al., 1994) that (4) has a unique solution of the form

 (5)
(5)
where  and n are found from the set of equations
and n are found from the set of equations
 (6)
(6)
Excluding n, we arrive at a set of equations for only 
 (7)
(7)
From (7) we can see that  depends only on ratios of the coordinates of
depends only on ratios of the coordinates of  . Thus if we introduce the quantities
. Thus if we introduce the quantities
 (8)
(8)
then they also depend only on ratios of the coordinates of  .
.
Theorem 2 (Weak theorem on the Maximum of Species Abundances for the Problem with Equalities). If  belongs to the interior of the cone
belongs to the interior of the cone  , then
, then  (8) has a global maximum at
(8) has a global maximum at  .
.
(A proof is presented in Appendix 2.) ¦
Remark: It can be shown that the requirement that  belong to the interior of K, can be rejected, so that, for all
belong to the interior of K, can be rejected, so that, for all  , the function
, the function  (8) reaches its maximum at
(8) reaches its maximum at  (perhaps after an appropriate extension of a definition on the continuity). We do not need this fact in the present paper.
(perhaps after an appropriate extension of a definition on the continuity). We do not need this fact in the present paper.
Let us now study the connection between the problems (1) and (4) in more detail. Without loss of generality we put m =3 to avoid lengthy calculations. The unknown Lagrange multipliers in (5) — (7) will be denoted x, y and z.
Consider the surface N in IR3 with coordinates x, y, z determined by the equation  . Then
. Then  IR3+ is some bounded nonempty set. Consider the image of V due to a mapping of N into IR2 with coordinates
IR3+ is some bounded nonempty set. Consider the image of V due to a mapping of N into IR2 with coordinates  specified by (7)
specified by (7)  . As has been shown in the proof of Theorem 2, this is a one-to-one mapping, differentiable infinitely many times along with its inverse (
. As has been shown in the proof of Theorem 2, this is a one-to-one mapping, differentiable infinitely many times along with its inverse ( diffeomorphism) onto the image. Therefore the image of V is a certain bounded set
diffeomorphism) onto the image. Therefore the image of V is a certain bounded set  in IR2; in IR3 the set
in IR2; in IR3 the set  corresponds to a certain cone
corresponds to a certain cone 
 IR° 3+
IR° 3+  (The fact that
(The fact that  is obtained as a consequence of the proof of Theorem 2: in the mapping (7), the image of the surface N is such a domain T in R2 with the coordinates t1 and t2 that
is obtained as a consequence of the proof of Theorem 2: in the mapping (7), the image of the surface N is such a domain T in R2 with the coordinates t1 and t2 that  belongs to the interior of K if and only if
belongs to the interior of K if and only if  with
with  and
and  belong to T. And the set Vt, to which
belong to T. And the set Vt, to which  corresponds, is the image of the set V in the mapping (7). And since V Ì N, Vt Ì T as well.)
corresponds, is the image of the set V in the mapping (7). And since V Ì N, Vt Ì T as well.)
Now let  . Then for this
. Then for this  , by construction of
, by construction of  , Eqs. (6) have a (unique) solution with
, Eqs. (6) have a (unique) solution with  . But then this solution of (6) will be a solution of (3) for the same
. But then this solution of (6) will be a solution of (3) for the same  ; moreover, as the problem (3) has a unique solution, it can have no other solution for this
; moreover, as the problem (3) has a unique solution, it can have no other solution for this  . Thus for
. Thus for  the problems (1) and (4) are equivalent (have the same solutions).
the problems (1) and (4) are equivalent (have the same solutions).
The cone  has three two-dimensional faces and three one-dimensional ribs, corresponding to intersections of N with the faces and ribs of IR3+. Let
has three two-dimensional faces and three one-dimensional ribs, corresponding to intersections of N with the faces and ribs of IR3+. Let  belong to the face of
belong to the face of  corresponding to x =0. Then, for this
corresponding to x =0. Then, for this  , the solutions to the set of equations (6) with
, the solutions to the set of equations (6) with  are solutions to the set of equations
are solutions to the set of equations

Consequently,  , found from (5), is a solution not only to (4) with J ={1; 2; 3} and a given
, found from (5), is a solution not only to (4) with J ={1; 2; 3} and a given  , but also to (4) with J = {2; 3} and
, but also to (4) with J = {2; 3} and  Moreover, let
Moreover, let  IR° 3+ be such that
IR° 3+ be such that  . Then, for this
. Then, for this  x = 0, n, y and z obtained from (6) and (7), form a solution to (3) and this solution is unique. Thus, for such
x = 0, n, y and z obtained from (6) and (7), form a solution to (3) and this solution is unique. Thus, for such  , (1) is equivalent to (4) with J ={2; 3} and
, (1) is equivalent to (4) with J ={2; 3} and  .
.
A similar analysis carried out for any m (Levich et al., 1994) shows that the following result is correct:
Theorem 3 (Stratification Theorem). The whole space IR° m+  IRm
IRm is a union of
is a union of  nonintersecting subsets
nonintersecting subsets  These subsets are in a one-to-one correspondence with nonempty subsets J of the set
These subsets are in a one-to-one correspondence with nonempty subsets J of the set  : if some vector
: if some vector  belongs to
belongs to  , then the problem (1) for this
, then the problem (1) for this  is equivalent to the problem
is equivalent to the problem
 ¦
¦
The meaning of Theorem 3 is that, in the set SJ, the constraint inequalities in the problem (1) with the numbers jÏ J may be discarded, while the remaining ones may be replaced by constraint equalities. In other words, if the nutrient amounts vector  belongs to SJ, then only the substances numbered jÎ J take part in the community structure formation at the stationary stage of the polyculture growth. These substances are entirely consumed, and one may leave the remaining ones without consideration.
belongs to SJ, then only the substances numbered jÎ J take part in the community structure formation at the stationary stage of the polyculture growth. These substances are entirely consumed, and one may leave the remaining ones without consideration.
A typical stratification picture of IR° m+ for m =2 and m =3 is presented in Figs. 1 and 2. The qualitative picture for  is the same. There exists a cone
is the same. There exists a cone  such that inside it the problem (1) is equivalent to (4) with
such that inside it the problem (1) is equivalent to (4) with  . Stratification of the whole IR° m+ is obtained with the aid of hypersurfaces originating from the ribs of
. Stratification of the whole IR° m+ is obtained with the aid of hypersurfaces originating from the ribs of  . As shown above, the cone
. As shown above, the cone  is entirely determined by the quantities
is entirely determined by the quantities  .
.
Let us now introduce the quantities
 , (9)
, (9)
where  and
and  are those from (2). (In our model
are those from (2). (In our model  have the meaning of relative abundances.) We see that
have the meaning of relative abundances.) We see that  depend only on
depend only on  and therefore on the ratios of the components of
and therefore on the ratios of the components of  . (Note that
. (Note that  (9) coincide with
(9) coincide with  (8) only for
(8) only for  .)
.)
Theorem 4 (Theorem on a Maximum of Abundances of Species for a Problem with Inequalities). The quantities  (9) reach their maximum at
(9) reach their maximum at  where
where  and
and 
 .
.
Proof: the case m =2 has been investigated earlier (Levich et al., 1993a). Here we will consider the case m =3. The general case differs from it only by more cumbersome technical details.
Let us first of all again note that  (9) in
(9) in  coincide with
coincide with  (8), since, by definition of
(8), since, by definition of  , the problems (1) and (4) are equivalent in it. The values of
, the problems (1) and (4) are equivalent in it. The values of  in the remaining part of IR° 3+ are determined by the values of
in the remaining part of IR° 3+ are determined by the values of  on the boundary of
on the boundary of  , because if we wish to find the value of
, because if we wish to find the value of  at a certain point
at a certain point  , belonging, for instance, to the domain IV (Fig. 2), then we must act in the following way. Take a straight line
, belonging, for instance, to the domain IV (Fig. 2), then we must act in the following way. Take a straight line  passing through this point. According to the aforesaid, along this line within the domain IV the value of
passing through this point. According to the aforesaid, along this line within the domain IV the value of  remains constant (it is obtained from (4) with J = {2; 3} and
remains constant (it is obtained from (4) with J = {2; 3} and ). Therefore, finding the intersection point of this straight line and a face of
). Therefore, finding the intersection point of this straight line and a face of  (this point is unique due to uniqueness of the solutions of (1) and (4)) and determining the value of
(this point is unique due to uniqueness of the solutions of (1) and (4)) and determining the value of  at this intersection point, we shall just find the value of
at this intersection point, we shall just find the value of  in
in  .
.
Thus if for some  we have
we have  and
and  , then our theorem follows directly from theorem 2.
, then our theorem follows directly from theorem 2.
Therefore we will consider the case  . Without loss of generality we assume that
. Without loss of generality we assume that  belongs to the set II (Fig. 2). Other cases are studied quite analogously.
belongs to the set II (Fig. 2). Other cases are studied quite analogously.
As  depends only on a ratio of the coordinates of
depends only on a ratio of the coordinates of  , let us consider a section of Fig. 2 by the plane
, let us consider a section of Fig. 2 by the plane  = const (Fig. 3). The coordinates of Fig. 3 are
= const (Fig. 3). The coordinates of Fig. 3 are  and
and  and the domains are labelled in the same way as in Fig. 2. The point A corresponds to a straight line with the direction vector
and the domains are labelled in the same way as in Fig. 2. The point A corresponds to a straight line with the direction vector  and has the coordinates
and has the coordinates  and
and  .
.
In II (1) is equivalent to (4) with J ={1; 2}, therefore  in II depends on
in II depends on  and, according to Theorem 2, reaches its maximum at
and, according to Theorem 2, reaches its maximum at  , i.e., on the straight line l passing through A. Thus in II
, i.e., on the straight line l passing through A. Thus in II  , where B is any point from II.
, where B is any point from II.
In V and VI (1) is equivalent to (4) with J ={1} and J ={2}, therefore the values of  in V and VI are constants determined by the values of
in V and VI are constants determined by the values of  on the boundaries of V with II and VI with II. Consequently,
on the boundaries of V with II and VI with II. Consequently,  , where B is any point from II, V or VI.
, where B is any point from II, V or VI.
Now consider the domain III. In this domain  depends on
depends on  and is therefore constant along the straight lines
and is therefore constant along the straight lines  const which lie in this domain. Let the straight line
const which lie in this domain. Let the straight line  lie in III (Fig. 3). Then
lie in III (Fig. 3). Then  for any point B from III, where C is the intersection point of
for any point B from III, where C is the intersection point of  with the boundary between I and III (Fig. 3) (this latter point is unique — see above). In I
with the boundary between I and III (Fig. 3) (this latter point is unique — see above). In I  depends on
depends on  and
and  (see the proof of Theorem 2). Therefore
(see the proof of Theorem 2). Therefore

However, 
 and
and  (see Appendix 2) and therefore
(see Appendix 2) and therefore

Consequently, for  and
and  we have
we have  (since
(since  , see Appendix 3). As a result,
, see Appendix 3). As a result,  , where D is the intersection point of the straight line
, where D is the intersection point of the straight line  and the boundary between I and II. However,
and the boundary between I and II. However, and therefore
and therefore  where B lies in II, III, V, VI or VII (the latter since in VII
where B lies in II, III, V, VI or VII (the latter since in VII  is a constant with a value equal to the value of
is a constant with a value equal to the value of  at the boundary between III and VII).
at the boundary between III and VII).
A maximum of  in IV is reached on the boundary between VII and IV if the straight line
in IV is reached on the boundary between VII and IV if the straight line  lies in VII and on the boundary between IV and VI if it lies in VI (this follows from Theorem 2 for J ={2; 3}). Moreover, if the line
lies in VII and on the boundary between IV and VI if it lies in VI (this follows from Theorem 2 for J ={2; 3}). Moreover, if the line 
 lies in IV, we apply a similar argument and make use of the fact that in I
lies in IV, we apply a similar argument and make use of the fact that in I  . In all cases
. In all cases  , where B lies in II — VII.
, where B lies in II — VII.
The cases when the straight line  lies in V and VII is studied in a similar way.
lies in V and VII is studied in a similar way.
Thus we have shown that  for all B lying in II — VII (including the boundaries).
for all B lying in II — VII (including the boundaries).
Let us finally consider  in I. I is a compact set, therefore
in I. I is a compact set, therefore  reaches its maximum value within it. This maximum cannot be interior, since in such a case it would be a local maximum of
reaches its maximum value within it. This maximum cannot be interior, since in such a case it would be a local maximum of  (8) in (4) with J ={1; 2; 3}. However, it was shown in Theorem 2 that
(8) in (4) with J ={1; 2; 3}. However, it was shown in Theorem 2 that  either has a unique maximum in the interior of K for
either has a unique maximum in the interior of K for  (if
(if  lies inside K), or has no maximum at all. Therefore a maximum of
lies inside K), or has no maximum at all. Therefore a maximum of  in I can occur only on its boundary. But we have already considered this case.
in I can occur only on its boundary. But we have already considered this case.
The theorem is entirely proved. ¦
Comment: note that in a problem with inequalities a maximum is not strict, unlike a problem with equalities (Theorem 2). It is strict only in the case when  lies inside
lies inside  .
.
A typical picture of the behaviour of  for the case of two resources is presented in Fig. 4.
for the case of two resources is presented in Fig. 4.
4. Biological analysis and results. What is the biological meaning of the results obtained?
1. Theorem 1 tell us that a stationary phase of an batch culture is uniquely determined by the number of resources in the medium  and is described by (2):
and is described by (2):

where n and  are functions of
are functions of  , while
, while  is the vector of demands of the i-th species. We call this formula the species structure formula.
is the vector of demands of the i-th species. We call this formula the species structure formula.
The species structure formula qualitatively explains the origin of rank distributions of population abundances  in communities. Empirical rank distributions possess a sharply descendant concave form, well approximated by exponential (Motomura, 1932) or hyperbolic (Fedorov et al., 1977) functions. According to the species structure formula, the form of rank distributions of abundances depends on rank distributions of species' demands
in communities. Empirical rank distributions possess a sharply descendant concave form, well approximated by exponential (Motomura, 1932) or hyperbolic (Fedorov et al., 1977) functions. According to the species structure formula, the form of rank distributions of abundances depends on rank distributions of species' demands  . For instance, in the case of single-factor limitation by environmental resources, if the demands distribution is linear,
. For instance, in the case of single-factor limitation by environmental resources, if the demands distribution is linear,  , the abundances distribution turns out to be exponential, while under a logarithmic demands distribution,
, the abundances distribution turns out to be exponential, while under a logarithmic demands distribution,  , it is hyperbolic. These single-factor laws have been verified for phytoplankton communities of the White Sea (Levich, 1980).
, it is hyperbolic. These single-factor laws have been verified for phytoplankton communities of the White Sea (Levich, 1980).
The adequacy of the species structure formula was investigated by the authors in experiments with laboratory algocoenoses.
In an experiment with a polyculture of 10 species of green microalgae (Chlorella vulgaris (Bejerink.), Scotiella nivalis (Fritsch.), Chromochloris cinnoborina (Chodat.), Scenedesmus quadricauda (Turp.), Sc. bijugatus (Lagerh.), Sc. obliquus (Kruger.), Ankistrodesmus acicularis (Korschik.), A. braunii (Brunnth.), Stichococcus mirabilis (Lagerh.) and Chlamydomonas humicola (Luksch.)), for 70 days and in several runs, the demands of the species for mineral phosphorus, the species numbers and biomasses, the primary production and the concentrations of mineral forms of nitrogen and phosphorus were measured. The algae grew in an batch regime without bubbling, on the Beneke medium. The content of biogenic elements in the medium was chosen is such a way that the algal development be restricted by environmental phosphorus. The cell numbers distribution at a stationary growth phase was described by the species structure formula with a multiple correlation factor of 0.97 (Levich et al., 1986b). This series of experiments, along with sixteen more series, was analyzed using the rank sample adequacy criteria and with the aid of cell quotas calibration (Zamolodchikov et al., 1993). The series of experiments differed from each other by the choice of taxa (from four to ten in a polyculture) and by initial nitrogen and phosphorus concentrations in the medium. When the growth is restricted by a single resource, according to the species structure formula, the place of a species in the sequence of abundances, ranged by decreasing numbers, is entirely determined at the stationary phase by the species' cell quota rank with respect to the limiting factor (the greatest abundance corresponds to the smallest cell quota). It has been attempted to select, within the experimental error, a unique set of ranks of experimentally measured cell quotas in such a way as to display the experimental ranks of abundances already in all series where the growth was restricted by a single resource. It was successfully done with Spearman's rank correlation factor in the range of 0.92 to 1.00. For the series with more than one limiting factor, the verification procedure for the species structure formula consisted in the following. Based on the species abundances in a pair of series, the nitrogen and phosphorus cell quotas were calculated; then, for all the other series, the species abundances were calculated, using the species structure formula with the above calibrated quotas, and compared with their experimental values. The comparison has shown that the calculated values of abundances coincided with the experimentally measured ones within the experimental errors.
We find it necessary to note as well that, although by the species structure formula  for all i at the stationary stage, this is not in conflict with the classical competitive exclusion principle (the Gause principle) claiming that the number of species, coexisting in equilibrium for an infinitely long time, cannot exceed the number of resources necessary for their existence. Indeed, in our model we have neglected the lysis, which is justified only within a finite time interval from the growth beginning and therefore, as already mentioned, our stationary state exists only for a limited period of time. We do not mean any infinitely lasting coexistence.
for all i at the stationary stage, this is not in conflict with the classical competitive exclusion principle (the Gause principle) claiming that the number of species, coexisting in equilibrium for an infinitely long time, cannot exceed the number of resources necessary for their existence. Indeed, in our model we have neglected the lysis, which is justified only within a finite time interval from the growth beginning and therefore, as already mentioned, our stationary state exists only for a limited period of time. We do not mean any infinitely lasting coexistence.
2. According to our model, the relative abundances of species depend only on the nutrient amounts ratio. On this basis, we have undertaken an attempt to control the species compositions of artificial and natural algocoenoses by varying the initial concentration ratio of nitrogen N and phosphorus P in the nutrient medium. The results of species growth in the condition of batch culture were estimated by their partial contribution to the total numbers in the period when all the species entering into the polyculture had reached their stationary growth phase (Levich and Bulgakov, 1993).
In the first series of experiments we used two media with N:P =3.5 (n =11 mg/l and P =3 mg/l) and N:P =20 (N =50 mg/l and P =2.5 mg/l) and studied the competition between algae Protococcales: Scenedesmus quadricauda (Turp.) Breb. and Chlorella vulgaris Beyer. The results are presented in Fig.5a. In the second series of experiments we took S. quadricauda and Aukistrodesmus falcatus (Corda) Ralfs and the media with N:P =1.3 (N =4 mg/l, P =3 mg/l) and N:P =57 (N =34 mg/l, P =0.6 mg/l). The results are shown in Fig. 5b. We see that S. quadricauda prefers high nitrogen to phosphorus ratios, whereas for Ch. vulgaris and A. falcatus lower ones are preferable.
Similar experiments were performed with natural phytoplankton in vitro. When working with natural multispecies algocoenoses, the circle of problems to be solved widens. There appears an opportunity to analyze the nutrient ratio effect on batch separate phytoplankton species and their systematic groups.
In the experiments of Levich et al. (1992), studying the influence of different ratios of mineral N and P forms upon the phytoplankton species structure by batch culture of a natural algocoenosis, water from a fish-breeding pond was placed in flasks, where mineral forms of N and P were added in different weight combinations, taking into account the natural background: N:P=2.5, 20, 50 and 100. In the course of the experiment the flasks were kept in outdoor air. The final biomass was analyzed both by big phytoplankton taxa (the Protococcales and Volvox orders and the divisions of green, diatome and blue-green algae) and on the level of genera and species.
It turned out that big nitrogen to phosphorus ratios (20 to 50) stimulated the development of green algae, for diatoms the N:P values from 5 to 20 were optimal, while blue-green algae develop best at N:P from 2 to 5.
In the restricting inequalities of the problem (1) the quantity  may be approximately considered to be equal to the so-called minimal cell quota of the species i with respect to the element k, i.e., such a level that if the content of the element k in a cell of the species i is lowered, the further cell fission becomes impossible. Using a special hydrobiological methodology, we calculated the minimal cell quotas in nitrogen and phosphorus for a number of algal species (Levich and Artyukhova, 1991). Unfortunately, the cellular quota measurement methods yield significant errors and therefore a direct verification of the species structure formula, by a substitution of measured quota values to the problem (1) and a comparison of the obtained solution (2) with experiment, seems to be impossible. We used the quota values, which we had measured, in order to compare the ratios of these quotas with optimal N:P ratios obtained in the experiment described above. For Ankistrodesmus falcatus, the nitrogen quota was (0.4± 0.08)× 10-9 mg/cell, the phosphorus quota was (0.04± 0.01)× 10-9 mg/cell and therefore the quota ratio was within the range of 6 to 16. For Chlorella vulgaris, the nitrogen quota was (0.9± 0.2)× 10-9 mg/cell, the phosphorus one (0.05± 0.01)× 10-9 mg/cell, and the quota ratio was between 12 and 26. For Scenedesmus quadricauda the nitrogen quota was (3.3± 0.36)× 10-9 mg/cell, the phosphorus one less than 0.05× 10-9 mg/cell, therefore the quota ratio was greater than 59. Thus we can conclude that, although the large errors of cellular quota measurements do not allow one to speak of a quantitative correspondence between experiment and the model, there is a qualitative agreement of our experimental data with the theorem on maximum species abundances.
may be approximately considered to be equal to the so-called minimal cell quota of the species i with respect to the element k, i.e., such a level that if the content of the element k in a cell of the species i is lowered, the further cell fission becomes impossible. Using a special hydrobiological methodology, we calculated the minimal cell quotas in nitrogen and phosphorus for a number of algal species (Levich and Artyukhova, 1991). Unfortunately, the cellular quota measurement methods yield significant errors and therefore a direct verification of the species structure formula, by a substitution of measured quota values to the problem (1) and a comparison of the obtained solution (2) with experiment, seems to be impossible. We used the quota values, which we had measured, in order to compare the ratios of these quotas with optimal N:P ratios obtained in the experiment described above. For Ankistrodesmus falcatus, the nitrogen quota was (0.4± 0.08)× 10-9 mg/cell, the phosphorus quota was (0.04± 0.01)× 10-9 mg/cell and therefore the quota ratio was within the range of 6 to 16. For Chlorella vulgaris, the nitrogen quota was (0.9± 0.2)× 10-9 mg/cell, the phosphorus one (0.05± 0.01)× 10-9 mg/cell, and the quota ratio was between 12 and 26. For Scenedesmus quadricauda the nitrogen quota was (3.3± 0.36)× 10-9 mg/cell, the phosphorus one less than 0.05× 10-9 mg/cell, therefore the quota ratio was greater than 59. Thus we can conclude that, although the large errors of cellular quota measurements do not allow one to speak of a quantitative correspondence between experiment and the model, there is a qualitative agreement of our experimental data with the theorem on maximum species abundances.
Keeping in mind the dependence of relative species abundances on the biogenic element ratios and the theorem on maximum species abundances, we undertook an attempt of purposeful control over the structure of a natural phytoplankton community in situ. We studied the influence of the N:P ratio upon natural phytoplankton composition in the water of experimental fish-breeding ponds (with areas of 0.25 to 3.5 hectares) in the delta of the Volga river (Levich and Bulgakov, 1992) for three years (1987-1989). The obtained results were in agreement with the expectations. The N:P ratio change in the pond water from 4–5 to 25–30 by changing the fertilizing system led to an increased abundance of green algae, whose calculated nitrogen to phosphorus quota ratio is high, while the abundance of the blue-greens, with a low quota ratio, simultaneously decreased.
The influence of biogenic element ratio upon relative species abundances and consequently upon the algocoenosis structure was repeatedly mentioned as well by many other authors (see for instance Kilham, 1986; Blomqvist et al., 1989; Klapwijk, 1990; Bulgakov and Levich, 1995). Some of them considered the problem of connection between optimal biogenic element ratios and the species' demand ratios with respect to these elements. Thus, Rhee and Gotham (1980) found optimal N:P ratios for nine algal species in a medium and emphasized that they correspond to their demands for these elements. This is also in agreement with the theorem on maximum species abundances.
3. The biological meaning of the stratification theorem (Theorem 3) is also closely related to the ecological conception of limiting factors.
The terms “limitation” and “limiting factor” have firmly entered into the conceptual basis of modern ecology. There is no universally adopted strict definition of a limiting factor, but in concrete situations one says that certain factors are limiting if just these factors, in a sense specified by the context, entirely determine the ecosystem state. We would like to stress here that factoral ecology distinguishes the environmental factors which might be called physiological (such as the temperature and the pH value) and the resource ones (the energetic and substrate components of nutrition). The laws of limitation are valid solely for the resource factors.
For a situation similar to batch culture, i.e., such that a biosystem is developing in a medium at the expense of resources whose quantity is specified at a certain initial time instant and whose inflow from outside is absent in the process of development, we believe that it is natural to introduce the following definition (Levich et al., 1993b). We call a resource factor a limiting factor if, when the growth of a biosystem (e.g., a population or a community) has stopped, addition of such a factor into the environment (e.g., into the soil or the water) leads to recommenced growth.
If the two conditions are fulfilled: first, all the physiological factors are tolerant and, second, all the resource factors are taken into consideration, then one can be convinced that the growth termination is caused by exhausting some (at least one) environmental resources consumed by the community (if the threshold concentrations, remaining in the environment, are neglected). (Recall that we regard the resources to be perfectly complementary.) Assume that at the moment when the growth stops we add some amount of the resources to the medium. It is evident that the addition of those of them, which had not been entirely consumed, will not resume the growth, i.e., they cannot be limiting. Unlike that, an addition of those substances which had been previously entirely consumed, will remove the cause of the growth termination. Recall that in the context under consideration we are interested only in the causes of stopping connected with resource factors. In the above argument it is implicitly meant that the living nature obeys the principle of extended self-reproduction of organisms in the absence of factors limiting the growth. This principle does not allow the over-organism systems to stop their growth when there are unexhausted resources. Thus there appears another definition of limiting factors, equivalent to the above one: these are resources which are entirely consumed from the environment (up to threshold concentrations).
Taking this as a basis, we can formulate our stratification theorem in the form of the limiting link rule:
The space m of the consumed environmental resources splits (stratifies) into  nonintersecting domains; each domain corresponds to a unique set of consumed resources which (and only these ones) in this domain:
nonintersecting domains; each domain corresponds to a unique set of consumed resources which (and only these ones) in this domain:
- are entirely consumed (i.e., are limiting in the sense of our definition);
- entirely determine the stationary state of the community (i.e., the abundances of all species of the community at the stationary phase depend only on the resources from this set).
To make clear the connection between the limiting link rule and the classical conceptions, let us consider the case of a single-species community. Strictly speaking, this case is not included in our model because  for w=1 and the extremal problem (1) loses its meaning. However, the case w=1 does not require any extremal principle at all since the conservation laws are written in the form
for w=1 and the extremal problem (1) loses its meaning. However, the case w=1 does not require any extremal principle at all since the conservation laws are written in the form
 , (10)
, (10)
where x is the abundance of the single species and  is its demand for the k-th resource (see the restricting inequalities in (1)). On the basis of the principle of extended self-reproduction of organisms in the absence of factors limiting the growth (see above) we conclude that at the growth termination moment at least one of the inequalities (10) becomes an equality and hence defines a limiting link. It is easily seen that the limiting link rule is entirely preserved in this case: given
is its demand for the k-th resource (see the restricting inequalities in (1)). On the basis of the principle of extended self-reproduction of organisms in the absence of factors limiting the growth (see above) we conclude that at the growth termination moment at least one of the inequalities (10) becomes an equality and hence defines a limiting link. It is easily seen that the limiting link rule is entirely preserved in this case: given  , those factors
, those factors  will be limiting, for which
will be limiting, for which  (there can be several such factors). Thus we virtually obtain the classical principle of minimum (Liebig, 1840; Leon and Tumpson, 1975).
(there can be several such factors). Thus we virtually obtain the classical principle of minimum (Liebig, 1840; Leon and Tumpson, 1975).
Our limiting link rule can be thus treated as a natural generalization of the classical principle of minimum to a situation when a multispecies community consumes a number of perfectly complementary resources.
The existence of domains where different sets of environmental resources are limiting removes an opposition between Liebig's minimum principle and Mitscherlich's “principle of joint action of factors” claiming that the state of a community depends on the level of all environmental resources (Mitscherlich, 1925).
There exist experimental materials which adequately illustrate the limiting link rule. Thus, an experiment with laboratory algocoenoses (Levich and Bulgakov, 1993; Levich and Lichman, 1992), for laboratory communities of 2, 3, 4 and 8 species in media with varied nitrogen and phosphorus concentrations revealed domains where separately nitrogen or phosphorus were limiting factors and others where batch of them together played this role.
The methodology of these experiments assumes growth of a laboratory community consisting of several phytoplankton species up to a stationary phase under standard controlled conditions. The media for such growing were composed in such a way as to guarantee sufficiency of all nutrients except maybe nitrogen and phosphorus.
A stationary phase having been achieved, the polycultures were transferred to several different flasks and in each of them conditions were provided for evident removal of limitation from nitrogen or phosphorus, or their combination, with preserved conditions with respect to all other factors. Resumed growth in some of these flasks indicates the resources which had caused the growth stopping.
Due to the large errors of cellular quota determination, it is difficult to compare the positions of different limitation domains in the experiment with our calculations of these positions according to (7); however, one can speak of a qualitative agreement, namely, the existence of domains with different limitation types in the resource space.
We would also note that the data referred to by Rhee (1982) in his review clearly indicate the existence of domains with different limitation types, which agrees with the theory as well.
Another interesting consequence of the stratification theorem refers to the important biotechnological problem of environmental pollution utilization. The problem is to choose a community of microorganisms for a given set of pollution in such a way that the algocoenosis, in the process of its development, consume the whole bulk of pollution. According to the stratification theorem, this is achieved if the vector  lies in the cone
lies in the cone  where the problem with equalities (4) and that with inequalities (1) are equivalent (see Section 3). Since the cone
where the problem with equalities (4) and that with inequalities (1) are equivalent (see Section 3). Since the cone  is uniquely determined by the demands
is uniquely determined by the demands  (see (7)), the utilization problem reduces to selecting such species with the demands
(see (7)), the utilization problem reduces to selecting such species with the demands  that for given
that for given  the set of equations (7) have a solution with
the set of equations (7) have a solution with  . A more detailed presentation of this problem is given in the paper by Zamolodchikov and Levich (1992).
. A more detailed presentation of this problem is given in the paper by Zamolodchikov and Levich (1992).
5. Conclusion. In our work we have attempted to apply the conditional optimization method to a complex biological system — an batch culture of a multispecies community of microorganisms growing on a multicomponent substrate. We started from the hypothesis that a stationary phase of this culture yields a maximum of a function characterizing the diversity of the community under some restrictions having the meaning of conservation laws. The obtained analytic results indicate that
- the vector of the amounts of environmental resources uniquely determines the abundances of all species of the community at the stationary phase;
- in different domains of the resource space the abundances depend on different resources, so that for any set of resources there exists a domain where the species abundances depend only on the resources from this set;
- the relative species abundances at the stationary phase depend only on ratios of amounts of environmental resources;
- the relative abundance of a given species is maximum when the ratios of environmental resource amounts are equal to the ratios of this species' demands for these resources.
A agreement of the analytic results with experimental data enables us to conclude that our situation presents an example of a successful application of the conditional optimization method in biology.
The application of extremal principles in general, and in particular the conditional optimization method, to biological problems encounters criticism from most different sides. One can read about the subject in more detail in Rosen's (1986) work. We would like to concentrate on two aspects of this criticism.
It is often believed that extremal principles, being applicable to conservative systems in physics, are unapplicable to dissipative, developing systems usually dealt with in biology. Herewith one forgets that, under certain conditions, for finite time intervals, some biological systems can be called conservative with good accuracy. Thus, in the situation considered here, that of polyculture growth under batch culture, the amount of any nutrient remains constant in the “polyculture plus environment” system at the growth stage, provided the lysis can be neglected. So we have certain analogues of the integrals of motion in mechanics. Therefore in this situation it is sufficient to apply adequately the conditional optimization method, originating from classical thermodynamics. That is what we tried to demonstrate.
Our second remark concerns the choice of a goal function, the one whose maximum or minimum distinguishes the only state (or a small number of states) related to reality from an infinite set of states specified by certain restrictions. This choice is at present a subject of a researcher's intuition rather than a result of some regular procedure. The latter is apparently the weakest point of the conditional optimization method. We are convinced, however, that it is quite correct to pose the problem of constructing a regular algorithm able to “produce” for a given piece of reality a goal function (or functional) which should appear in an optimization problem describing the situation under study. One of possible approaches to the construction of such an algorithm, based on category theory, is worked out by our group for recent years. Some concrete results have been obtained on this way. Unfortunately, a presentation of this approach and these results would lead us too far from the subject of the present paper, therefore we restrict ourselves to just mentioning it and point out, for an interested reader, more detailed papers by one of the authors (Levich, 1982, 1995).
The mathematical proofs of the theorems presented in this paper belong to V.L.Alexeyev, the problem statement and the biological materials to A.P.Levich.
We thank two anonymous referees for helpful comments.
Appendix 1
Lemma: For each  IR° m+
IR° m+  IRm
IRm a maximum of H(
a maximum of H( ) in problem (1) is achieved at xi > 0,
) in problem (1) is achieved at xi > 0,  .
.
Proof. Assume the contrary, i.e., that a maximum is reached at a point where some  . Without restricting the generality, we assume that at the maximum point
. Without restricting the generality, we assume that at the maximum point  …,
…, 
 …,
…,  . Then
. Then 
 .
.
Consider, for some e>0, the straight line  ,
,  , ...,
, ...,  ,
,  ,
,  If
If  , then, as soon as
, then, as soon as  , one obtains
, one obtains  .
.
Let now  be a maximum value of
be a maximum value of  in the problem (1). It is easily seen that
in the problem (1). It is easily seen that
 ,
,
and hence the straight line  (t) crosses the set of level H(
(t) crosses the set of level H( ) = C at
) = C at
 ,
,
while for t >t(e) we obtain H( (t)) >C.
(t)) >C.
Since for e>0 one has  and
and  , there exists such e1 >0 that for any e such that 0 <e<e1 we have
, there exists such e1 >0 that for any e such that 0 <e<e1 we have  . Therefore we can take some e2 (0 <e2 <e1) and find for it such
. Therefore we can take some e2 (0 <e2 <e1) and find for it such  >0 that
>0 that  >
>  >
> and therefore at the point
and therefore at the point

we have

while  >C, which is contrary to the definition of C. ¦
>C, which is contrary to the definition of C. ¦
Appendix 2
Theorem. If  belongs to the interior of the cone
belongs to the interior of the cone  , then j i(8) has a global maximum at
, then j i(8) has a global maximum at  , a>0.
, a>0.
Proof. Let us consider in more detail the set of equations (7) for finding the Lagrange multipliers  .
.
In the coordinates , …,
, …, the interior of the cone K corresponds to a certain bounded domain TÎ IR° +m-1
the interior of the cone K corresponds to a certain bounded domain TÎ IR° +m-1 IRm-1
IRm-1 , because
, because

for  from K.
from K.
If we now rewrite (7) in the form
 (A2.1)
(A2.1)
then, since for  from the interior of K the solution (4) exists and is unique (it is given by (5)). We obtain that (A2.1) determines a bijection of the surface N:
from the interior of K the solution (4) exists and is unique (it is given by (5)). We obtain that (A2.1) determines a bijection of the surface N:  to the domain T.
to the domain T.
This is actually quite sufficient for a proof of Theorem 2, but for what follows we need some analytic properties of this bijection, which we are now going to prove.
Let us first of all note that the first equation (A2.1) enables us to define the function  IRm-1 ® IR and, according to the implicit function theorem,
IRm-1 ® IR and, according to the implicit function theorem,  (IRm-1; IR).
(IRm-1; IR).
Lemma: the quadratic form, defined by the Hessian (matrix of second partial derivatives) of  , is positive-definite (i.e.,
, is positive-definite (i.e.,  is a strictly convex function).
is a strictly convex function).
Proof. Let  be an arbitrary nonzero vector, then a direct calculation gives
be an arbitrary nonzero vector, then a direct calculation gives

where  and
and  . Note that
. Note that  because
because  , i=1, …, m, j=1, …, w.
, i=1, …, m, j=1, …, w.
We are interested in the case when  , since otherwise the lemma is evidently valid. Therefore one can put
, since otherwise the lemma is evidently valid. Therefore one can put  >0,
>0,  , since otherwise it is sufficient just to introduce
, since otherwise it is sufficient just to introduce  .
.
Denoting now

we obtain the following chain of equalities and inequalities:

The inequalities 1) and 3) are evident, while 2) is a direct consequence of Hölder's inequality. But 3) turns to an equality if and only if  , but under this condition 2) becomes an equality only if
, but under this condition 2) becomes an equality only if  and
and  are proportional; but this is contrary to the condition
are proportional; but this is contrary to the condition  . The lemma is thus entirely proved. ¦
. The lemma is thus entirely proved. ¦
But it is easily seen that in (A2.1)  k=1, …, m-1 and therefore the Jacobian of the mapping
k=1, …, m-1 and therefore the Jacobian of the mapping  , specified by the last m-1 equations from (A2.1), where
, specified by the last m-1 equations from (A2.1), where  , is just the Hessian of
, is just the Hessian of  and is, in particular, nondegenerate on IRm-1. Therefore, according to the inverse function theorem, the mapping
and is, in particular, nondegenerate on IRm-1. Therefore, according to the inverse function theorem, the mapping 
 is a local C¥ diffeomorphism of N onto T.
is a local C¥ diffeomorphism of N onto T.
We ultimately obtain that (A2.1) specifies a C¥ diffeomorphism of N onto T.
All the aforesaid implies that the problem of finding a maximum of  , where
, where  ,
,  and
and  , is equivalent on the domain T to the following problem:
, is equivalent on the domain T to the following problem:
 (A2.2)
(A2.2)
Let us solve (A2.2) by the Lagrange method. The Lagrange function is
 .
.
The necessary conditions for an extremum  imply
imply

Excluding l, we have
 (A2.3)
(A2.3)
The set of equations (A2.3) coincides with (7) and therefore, for  lying in the interior of K, has a unique solution. An analysis of the Hessian of L shows that this solution yields a maximum. The theorem is entirely proved. ¦
lying in the interior of K, has a unique solution. An analysis of the Hessian of L shows that this solution yields a maximum. The theorem is entirely proved. ¦
Appendix 3
As was shown in Appendix 2, the set of equations (A2.1) determines the C¥ functions on the domain  . Proov the following properties of these functions.
. Proov the following properties of these functions.
Corollary. In all points of domain T takes place

Proof. According to the inverse function theorem
 .
.
But, as was proved in Appendix 2,  specifies a positive-definite quadratic form and has therefore a positive determinant, positive elements along the diagonal and positive principal minors (i.e., minors with the same row and column numbers). Therefore we obtain the following:
specifies a positive-definite quadratic form and has therefore a positive determinant, positive elements along the diagonal and positive principal minors (i.e., minors with the same row and column numbers). Therefore we obtain the following:  on the whole T for i=1, …, m -1. ¦
on the whole T for i=1, …, m -1. ¦
literature
Armstrong, R.A. and R. McGehee. 1980. Competitive exclusion. American Naturalist 115, 151-170.
Ballyk, M.M. and G.S.K. Wolkowicz. 1995. An examination of the thresholds of enrichment: a resourse-based growth model. J. Math. Biol. 33, 435-457.
Blomqvist, P., H. Olsson, H. Olofsson and O. Broberg. 1989. Enclosure experiments with low-dose additions of phosphorus and nitrogen in the acidified lake Njupfatet, Central Sweden. Int. Rev. Gesamt. Hydrobiol. 74, 611-631.
Brillouin, L. 1963. Science and information theory. New York: Acad. Press.
Bulgakov, N.G. and A.P. Levich. 1995. Biogenic elements in the environment and phytoplankton: ratio N:P as an independent factor to regulate the algocoenosis structure. Uspekhi Sovremennoy Biologii 115, 13-23 (in Russian).
Butler, G.I. and G.S.K. Wolkowicz. 1987. Exploitative competition in a chemostat for two complementary, and possibly inhibitory, resources. Math. Biosci. 83, 1-48.
Degermedzhy, A.G., V.A. Adamovich and V.N. Pozdyayev. 1989. On the cybernetics of bacterial communities: observations, experiments, and theory. Cybernetics and Systems 20, 501-541.
Droop, M.R. 1968. Vitamin B12 and marine ecology. IV. The kinetics of uptake, growth and inhibition in Monochrysis lutheri. J. Mar. Biol. Assoc. U. K. 48, 689-733.
Droop, M.R. 1973. Some thoughts on nutrient limitation in algae. J. Phycol. 9, 264-272.
Droop, M.R. 1983. 25 years of algae growth kinetics: a personal view. Bot. Mar. 26, 99-112.
Fedorov, V.D., E.N. Kondrik and A.P. Levich. 1977. A rank distribution of White Sea phytoplankton abundance. Doklady AN SSSR 236, 264–267 (in Russian).
Fredrickson, A.G. and G. Stephanopoulos. 1981. Microbial competition. Science 213, 972-979.
Gibbs, J.W. 1902. Elementary principles in statistical mechanics. New York: Longmans, Green&Co.
Haken, H. 1988. Information and self-organization: a macroscopic approach to complex systems. Berlin: Springer.
Intriligator M. 1971. Mathematical optimization and economic theory. New York: Prentice-Hall.
Jørgensen, S.E. 1980.
Lake managment. Oxford: Pergamon Press.
Karpouzas, I. 1986. Respiratory parameters. Minimization of the energy cost of breathing. Math. Biosci. 78, 1-20.
Ketchum, B.H. 1939. The absortion of phosphate and nitrate by illuminated cultures of Nitzschia closterium. Am. J. Bot. 26, 399-407.
Kilham, S.S. 1986. Dynamic of Lake Michigan natural phytoplankton communities in continuous cultures along a Si:P loading gradient. Can. J. Fish. and Aquat. Sci. 43, 351-360.
Klapwijk,S.P. 1990. Comparison of historical and recent data on hydrochemistry and phytoplankton in the Rijnland area (the Netherlands). Hydrobiologia 199, 87-100.
Kuenzler E.J. and B.H. Ketchum. 1962. Rate of phosphorus uptake by Phaedactylum tricornutum. Biol. Bull. Marine Biol. Lab., Woods Hall 123, 134-145.
Leon, J.A. and D.B. Tumpson. 1975. Competition between two species for two complementary or substitutable resources. J. Theor. Biol. 50, 185-201.
Levich, A.P. 1980. Structure of ecological communities. Moscow: Moscow University Press (in Russian).
Levich, A.P. 1982. Sets theory, the language of category theory and their applications in theoretical biology. Moscow: Moscow University Press (in Russian).
Levich, A.P., N.V. Revkova and N.G. Bulgakov. 1986a. The “consumption-growth” process in microalgal cultures and cells' demands for mineral nutrition components. In: Ecological Forecast. V.N. Maximov (ed.), pp. 132–139. Moscow: Moscow University Press (in Russian).
Levich, A.P., E.G. Liubimova and G.Sh. Martashvili. 1986b. Species structure and consumption of substrate-energy factors in laboratory algocoenoses. In: Ecological Forecast. V.N. Maximov (ed.), pp.69-103. Moscow: Moscow University Press (in Russian).
Levich, A.P. and V.I. Artyukhova. 1991. Measuring requirements of phytoplankton for environmental substrate factors. Biol. Bull. of the Academy of Sciences of the USSR 18(1), 86-93.
Levich, A.P. and N.G. Bulgakov. 1992. Regulation of species and size composition in phytoplankton communities in situ by N:P ratio. Russian J. of Aquatic Ecology 2, 149-159.
Levich, A.P. and E.G. Lichman. 1992. Model studies of an opportunity of directed structure change of a phytoplankton community. Zhurnal Obshchey Biologii 53, 689–703 (in Russian).
Levich, A.P., A.A. Khudoyan, N.G. Bulgakov and V.I. Artyukhova. 1992. On a possibility to control the species and size structure of a community in experiments with natural phytoplankton in vitro. Biologicheskiye Nauki 7, 17–31 (in Russian).
Levich, A.P. and N.G. Bulgakov. 1993. Possibility of controlling the algal community structure in the laboratory. Biol. Bulletin of the Russian Acad. Sciences 20(4), 457-464.
Levich, A.P., V.L. Alexeyev and S.Yu. Rybakova. 1993a. Optimization of the structure of ecological communities: model analysis. Biophysics 38, 903-911.
Levich, A.P., D.G. Zamolodchikov and V.L. Alexeyev. 1993b. Limiting link rule for multispecies community consuming essential resources. Zh. Obshch. Biol. 54, 271-286 (in Russian).
Levich, A.P., V.L. Alexeyev and V.A. Nikulin. 1994. Mathematical aspects of variation modelling in ecology of communities. Matematicheskoe modelirovanie 6(5), 55-76 (in Russian).
Levich, A.P. 1995. Time as variability of natural systems: ways of quantitative description of changes and creations of changes by substantial flows. In: On the Way to Understanding the Time Phenomenon: the Constructions of Time in Natural Sciences. Part 1. A.P. Levich (Ed.), pp.149-199. Singapore: World Scientific.
Levine, R.D. and M. Tribus (Eds.). 1979. The maximum entropy formalism. Cambridge, Mass.: MIT Press.
Lewis III, H.W., N.G. Goel and R.L. Thompson. 1988. Simulation of cellular compaction and internalization in mammalian embryo development-II. Models for spherical embryos. Bull. Math. Biol. 50, 121-142.
Liebig, J. 1840. Chemistry in its application to agriculture and physiology. London: Taylor & Walton.
Luenberger, D.G. 1984. Linear and nonlinear programming. 2d ed. Reading (Mass.): Addison-Wesley.
Ludwig, J.A. and J.F. Reynolds. 1988. Statistical ecology: a primer on methods and computing. New York: Wiley Corp.
Lurie, D., J. Valls and J. Wagensberg. 1983. Thermodynamic approach to biomass distribution in ecological systems. Bull. Math. Biol. 45, 869-872.
Mac Arthur, R.H. 1955. Fluctuations of animal populations and measure of community stability. Ecology 36, 533-536.
Margalef, R. 1951. A practical proposal to stability. Publ. De Inst. De Biol. Apl. Univ. De Barcelona 6, 5-19.
Menhinick, E.F. 1964. A comparison of some species-individuals diversity indices applied to samples of field insects. Ecology 48, 392-404.
Mitscherlich, E.A. 1925. Die Bestimmung des Dungerbedurfuisses der Bodens. Berlin: 2 Aufl. Darly.
Motomura, I. 1932. A statistical treatment of associations. Japan. J. Zool. 44, 379-383 (in Japanese).
Odum, E.P. 1983. Basic Ecology. V.2. Philadelphia: Saunders College Publ.
Odum, H.T., J.E. Cantlon and L.S. Kornicker. 1960. An organizational hierarchy postulate for the interpretation of species-individuals distributions, species entropy and ecosystem evolution and the meaning of a species variety index. Ecology 41, 395-399.
Prits, A.K. 1974. principle of stationary state of open system and population dynamic. Kaliningrad: Kaliningrad University Press (in Russian).
Rhee, G.-Yu. 1982. Effects of environmental factors and their interactions on phytoplankton growth. Adv. Microb. Ecol. 6, 33-74.
Rhee, G.-Yu. and I.J. Gotham. 1980. Optimum N:P ratios and coexistence of planktonic algae. J. Phycol. 16, 486-489.
Rosen, R. 1986. Optimality in Biology and Medicine. J. Math. Analysis and appl. 119, 203-222.
Schuster, S. and R. Heinrich. 1991. Minimization of intermediate concentrations as a suggested optimality principle for biochemical networks. I. Theoretical analysis. J. Math. Biol. 29, 425-442.
Tilman, D. 1982. resource competition and community structure. New Jersey: Princeton University Press.
Vavilin, V.A., V.B. Vasiliev and S.V. Rytov. 1993. Modelling of organic matter destruction by microorganisms community. Moscow: Nauka (in Russian).
Waltman P., S.P. Hubbel and S.B. Hsu. 1980. Theoretical and experimental investigation of microbial competition in continuous culture. In: Modelling and Differential equations. T. Burton (Ed.). New York: Murcel Dekker.
Wilhelm, T., E. Hoffmann-Klipp and R. Heinrich. 1994. An evolutionary approach to enzyme kinetics: optimisation of ordered mechanisms. Bull. Math. Biol. 56, 65-106.
Woodwell, G.M. and H.H.Smith (Eds.). 1969. Diversity and stability in ecological systems. Brookhaven Symp. Biol. V.22.
Zamolodchikov, D.G. and A.P. Levich. 1992. Selection of plankton alga species for complete utilization of polybiogenic load on a waterbody. Moscow University Biological Sciences Bulletin 47(3), 234-246.
Zamolodchikov, D.G., A.P. Levich and S.Yu. Rybakova. 1993. A study of the adequacy of a category-theoretic model of phytoplankton communities. Ecological Monitoring Problems and Ecosystem Modelling 15, 234–246 (in Russian).
 )
) (1)
(1)
 , while n and
, while n and  (3)
(3) , then lk = 0 and therefore we obtain from Eq. (2) that xi,
, then lk = 0 and therefore we obtain from Eq. (2) that xi,  (4)
(4) , where
, where 
 (5)
(5) (6)
(6)  (7)
(7) . As has been shown in the proof of Theorem 2, this is a one-to-one mapping, differentiable infinitely many times along with its inverse (
. As has been shown in the proof of Theorem 2, this is a one-to-one mapping, differentiable infinitely many times along with its inverse ( (The fact that
(The fact that 
 ¦
¦  , (9)
, (9) and
and  and
and  .
.  and, according to Theorem 2, reaches its maximum at
and, according to Theorem 2, reaches its maximum at  , i.e., on the straight line l passing through A. Thus in II
, i.e., on the straight line l passing through A. Thus in II  lie in III (
lie in III (

 and
and  we have
we have  (there can be several such factors). Thus we virtually obtain the classical principle of minimum (Liebig, 1840; Leon and Tumpson, 1975).
(there can be several such factors). Thus we virtually obtain the classical principle of minimum (Liebig, 1840; Leon and Tumpson, 1975).  , then, as soon as
, then, as soon as  be a maximum value of
be a maximum value of  ,
, ,
, , then j i(8) has a global maximum at
, then j i(8) has a global maximum at  , …,
, …, the interior of the cone K corresponds to a certain bounded domain TÎ IR° +m-1
the interior of the cone K corresponds to a certain bounded domain TÎ IR° +m-1
 (A2.1)
(A2.1)
 , since otherwise the lemma is evidently valid. Therefore one can put
, since otherwise the lemma is evidently valid. Therefore one can put  >0,
>0,  , since otherwise it is sufficient just to introduce
, since otherwise it is sufficient just to introduce 

 k=1, …, m-1 and therefore the Jacobian of the mapping
k=1, …, m-1 and therefore the Jacobian of the mapping  (A2.2)
(A2.2) .
.
 (A2.3)
(A2.3)
 .
. specifies a positive-definite quadratic form and has therefore a positive determinant, positive elements along the diagonal and positive principal minors (i.e., minors with the same row and column numbers). Therefore we obtain the following:
specifies a positive-definite quadratic form and has therefore a positive determinant, positive elements along the diagonal and positive principal minors (i.e., minors with the same row and column numbers). Therefore we obtain the following:  on the whole T for i=1, …, m -1. ¦
on the whole T for i=1, …, m -1. ¦